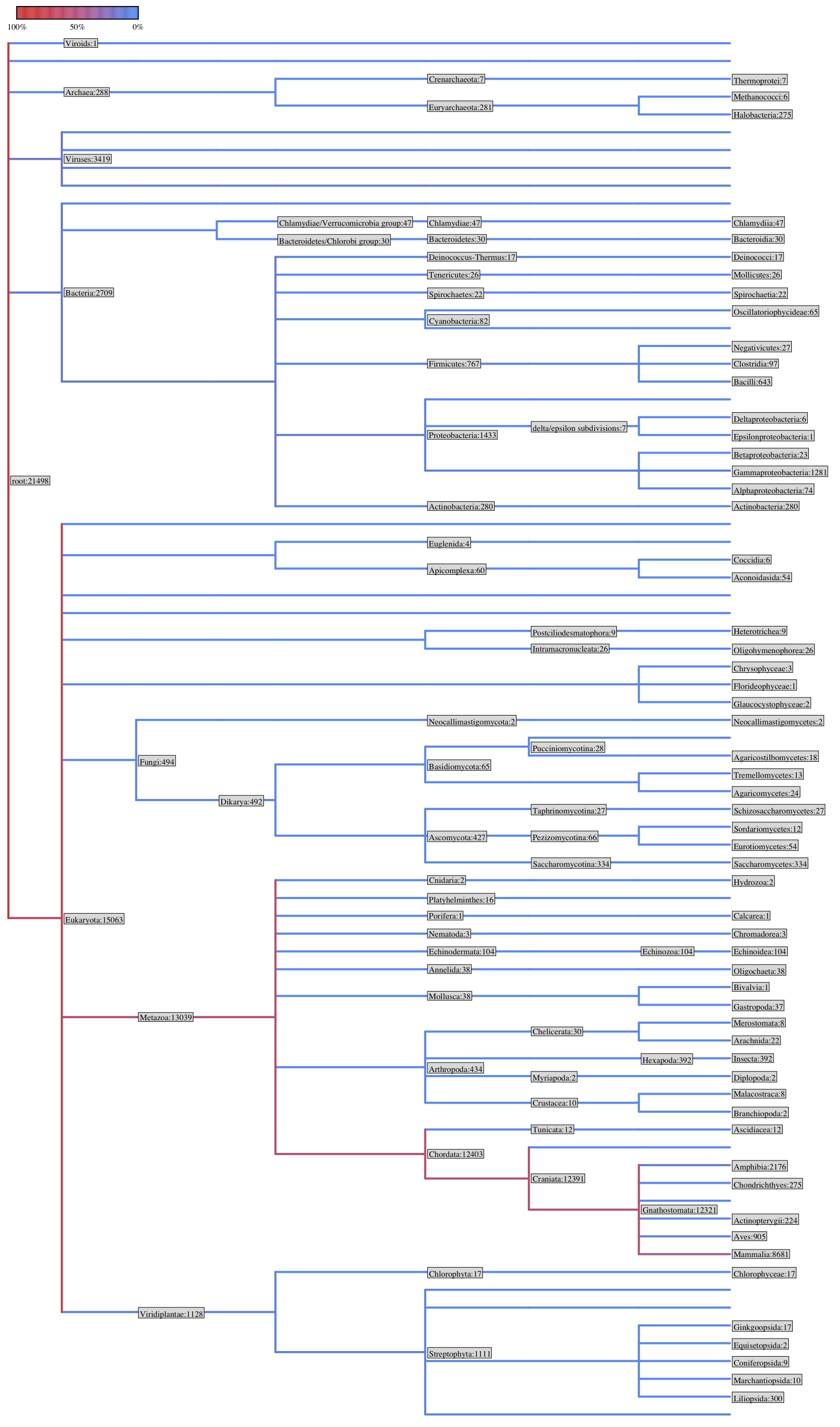
taxonomic representation of OlyO transcriptome v3 cdd blast through galaxy
- uploaded cdd blast file
- "fetch taxonomic data"
| Phylum |
Counts |
| Chordata |
12403 |
| Proteobacteria |
1433 |
| Streptophyta |
1111 |
| Firmicutes |
767 |
| Arthropoda |
434 |
| Ascomycota |
427 |
| Euryarchaeota |
281 |
| Echinodermata |
104 |
| Cyanobacteria |
82 |
| Basidiomycota |
65 |
| Apicomplexa |
60 |
| Chlamydiae |
47 |
| Annelida |
38 |
| Mollusca |
38 |
| Bacteroidetes |
30 |
| Tenericutes |
26 |
| Spirochaetes |
22 |
| Chlorophyta |
17 |
| Deinococcus-Thermus |
17 |
| Platyhelminthes |
16 |
| Crenarchaeota |
7 |
| Euglenida |
4 |
| Nematoda |
3 |
| Cnidaria |
2 |
| Neocallimastigomycota |
2 |
| Porifera |
1 |
March 27, 2014
Parameters on the RSeq Analysis:
-OlyO Transcriptome v2 as reference genome
-min read length fraction: 0.9
-max hits per read: 10
Parameters in Expression Analysis:
-unpaired two group comparison
-set expression value to unique gene reads
Info for annotating genome and location of GOSlim database
http://oystergen.es/query
https://github.com/uwescience/sqlshare/wiki/Workflow:-Annotating-Oyster-Genes
February 18, 2014
- found non-integer!!
- Olurida_trim_nodups_v2reads_contig_56245 102 0.015 (old)
- Olurida_trim_nodups_v2reads_contig_56245 1 3102 (corrected)
- working with RNA-Seq Analysis files for 108 male/female broodstock data and 106 male/female broodstock data <-- looking at unique gene reads
- converted CLC files into text files through iPython
- ran DESeq script on the new text files through R
- constructed data plots for each file
Plots:
Scatter of 106 male/female unique gene reads
Histogram of 106 male/female unique gene reads
Scatter of 108 male/female unique gene reads
Histogram of 108 male/female unique gene reads
data table for 106 Broodstock
data table for 108 Broodstock
January 28, 2014
- ran RNA-Seq Analysis on OlyO broodstock data
January 21, 2014
- All files in CLC moved to CLC_local_bird --> Hannah
- Uploaded male and female data to iPlant and Galaxy
- also uploaded OlyO transcriptome to iPlant and Galaxy
January 07, 2014
- Sequence ID, GOID, and SPID for swissprot OlyO data into excel files- includes data charts
- in files on eagle under folder named "Hannah"
- Olympia oyster transcriptome fasta file uploaded to CLC workbench under folder named "Hannah"
- filtered female 106A OlyO paired data through NGS import
- file NGS Raw Data --> Friedman Broodstock --> full
- filtered male 106A OlyO paired data through NGS import (same file location as above)
- filtered female 108A OlyO paired data through NGS import (same file location as above)
- filtered male 108A OlyO paired data through NGS import (same file location as above)
October 16, 2013
Many of the blasts were not fully completed so they must be redone.
Re-doing failed blasts on the following databases:
- refseq_protein (Oly Blast 1)
- env_nr (Oly Blast 5)
- uniprot_swissprot (Oly Blast 12)
October 10, 2013
I have been having issues with the vector and pdbaa databases not blasting.
October 9, 2013
(list last revised 10/10/2013)
Blast databases tried:
- cdd_delta
- env_nr
- est_others
- nr
- nt
- refseq_genomic
- refseq_protein
- refseq_RNA
- env_nt
- uniprot_swissprot
Blast databases not tried:
Databases with Issues
- vector
- pdbaa
- swissprot
October 3, 2013
Running some more data through blast- added to the list on October 1st.
Reminder: DIfferent Blasts
- blastn: search nucleotide database using nucleotide query
- tblastn: search a translated nucleotide database using a protein query
- blastx: search a protein database using a translate nucleotide query
- tblastx: search a translated nucleotide database using a translated nucleotide query
- blastp: search a protein database using a protein query
Remember- to find notebook on iPython type ipython notebook --pylab inline into the terminal
d-128-95-149-219:~ srlab$ cd /Volumes/web/scaphapoda/Hannah
d-128-95-149-219:Hannah srlab$ pwd
/Volumes/web/scaphapoda/Hannah
d-128-95-149-219:Hannah srlab$ ipython notebook -pylab inline
Google Doc of BLAST Databases
October 1, 2013
Continuing blasting Oly transcriptome against various databases in whale folder
Program blastx works for the following databases:
- refseq_protein (output in Oly_Blast_1)
- cdd_delta (output in Oly_Blast_2)
- env_nr (output in Oly_Blast_5)
- nr (output in Oly_Blast_8)
Program blastn works for the following databases:
- refseq_genomic (output in Oly Blast 4)
- est_others (output in Oly_Blast_7)
- refseqgene (output in Oly Blast 9)
- refseq_rna (output in Oly Blast 10)
September 26, 2013
Ran blast yesterday (September 25) on Olympia oyster data comparing against new databases.
Continuing blast sequences today.
September 24, 2013
Learning more about IPython and SQLshare
Changing | to tabs in IPy:
!tr '|' "\t" </Volumes/web/scaphapoda/Hannah/OlyO_trans_v3_swissprot.txt> /Volumes/web/scaphapoda/Hannah/OlyO_trans_v3_swissprot_mod.txt
Joining Tables in SQLshare:
SELECT * FROM
[wearh@washington.edu].[OlyO_trans_v3_swissprot_mod.txt]oly
left join
[sr320@washington.edu].[associations_uni_swisspro_012410]des
on
oly.Column3 = des.ID
September 18, 2013
Steven showed me how to run IPython and BLAST
What was ran:
!blastx -query /Volumes/web/scaphapoda/Hannah/Olurida_transcriptome_v3.fasta -db /Users/Shared/Apps/ncbi-blast_28/db/uniprot_sprot -out /Volumes/web/scaphapoda/Hannah/Olur_blast_sprot.txt -outfmt 6 -evalue 1E-5 -max_target_seqs 1 -num_threads 14
Program: blastx
Language: -query (file)
Database: -db (file: /Users/Shared/Apps/ncbi-blast_28/db/uniprot_sprot)
Output Location: -out (new file in .txt format)
Other Info: -outfmt 6 -evalue 1E-5 -max_target_seqs 1 -num_threads 14
September 5, 2013
Took tissue samples from breeding group SN 9 and SN 30.
Group SN 9**
- mortality: 7/20
- samples in this group labeled SN_26A,B,C through SN_38A,B,C
- mantle tissue in samples A and B
- adductor tissue samples in samples C
Group SN 30
- mortality: 7/18
- samples in this group labeled SN_39A,B,C through SN_49A,B,C
- mantle tissue in samples A and B
- adductor tissue samples in samples C
All of the breeding group oysters from NF, HL, and SN have been sampled. Tissue samples were placed in storage nine boxes and labeled according to the contents: NF_A, NF_B, NF_C, HL_A, HL_B, HL_C, SN_A, SN_B, and SN_C.
September 4, 2013
Took tissue samples from breeding group SN 15 and SN 21.
Group SN 15
- mortality: 7/20
- samples in this group labeled SN_1A,B,C through SN_13A,B,C
- mantle tissue in samples A and B
- adductor tissue samples in samples C
Group SN 21
- mortality: 5/17
- samples in this group labeled SN_14A,B,C through SN_25A,B,C
- mantle tissue in samples A and B
- adductor tissue samples in samples C
August 22, 2013
Took tissue samples from breeding group HL 19.
- mortality: 1/16
- samples in this group labeled HL_50A,B,C through HL_64A,B,C
- mantle tissue in samples A and B
- adductor tissue samples in samples C
August 15, 2013
Took tissue samples from breeding group HL 16.
- mortality: 1/18
- samples in this group labeled HL_33A,B,C through HL_49A,B,C
- mantle tissue in samples A and B
- adductor tissue samples in samples C
August 09, 2013
Took tissue samples from breeding group HL 28.
- mortality: 6/19
- samples in this group labeled HL_19A,B,C through HL_32A,B,C
- mantle tissue in samples A and B
- adductor tissue samples in samples C
Shells from breeding group HL 4 were dried, labeled corresponding the tissue sample number and placed in individual plastic bag.
August 08, 2013
Started taking samples from a new breeding group: Hood Canal (HL). Took mantle and adductor muscle tissue samples from breeding group HL 4.
- mortality: 2/20
- samples in this group labeled HL_1A,B,C through HL_18A,B,C
- mantle tissue in samples A and B
- adductor tissue samples in samples C
August 06, 2013
Sampled the last group of North Sound Fidalgo Bay. Oysters were frozen so they were placed in a water bath to thaw. Took mantle and adductor tissue samples from breeding group NF #?
- Samples are labeled as NF_60A,B,C through NF_78A,B,C
- mortality: 0/19
July 31, 2013
Took mantle and adductor tissue samples from breeding group NF 8.
- Samples are labeled as NF_41A,B,C through NF_59A,B,C
- mortality: 0/19
July 30, 2013
Oyster shells from group NF 14 were labeled by writing the group and sample number directly onto the shell once dry. All oyster shells from the breeding groups will continue to be labeled in this same manner.
Took mantle and adductor tissue samples from oysters in group NF 20.
- Samples are labeled as NF_21A,B,C through NF_40A,B,C
- mortality: 0/19
July 25, 2013
Since the commercial oyster mantle tissue samples were being stored in snap lid capsules, they needed to be better preserved so we separated the A and B samples into containers and vacuum sealed them.
Breeding Group NF14 Olympic Oyster Tissue Samples
Started collecting tissue samples from oyster breeding groups. The same methods as described previously were used to collect two samples of mantle tissue per oyster in addition to a sample of adductor muscle from each oyster. The mantle tissues were labeled as NF#A and NF#B, and all adductor tissue samples were labeled as NF#C. Breeding group NF14 contained 20 live oysters (all sampled) and zero dead/un-sampled oysters. The oyster shells from NF14 were labeled corresponding to the tissue sample number and left to dry out for future research.
July 23, 2013
Finished sampling the commercial Olympic oysters that were collected last December, 2012. There were 142 oysters that tissue was extracted from with two samples per oyster (labeled A and B).
July 19, 2013
Continued to collect mantle tissue samples from Olympic oysters using the same procedure as described on July 28, 2013. Collected 52 tissue samples and starting to get a better understanding of oyster anatomy.
July 18, 2013
First day in the lab!
Olympic Oyster Mantle Tissue Samples
Took mantle tissue of commercial raised adult Olympic oysters (Ostreola conchaphila) for DNA extraction. Oysters were sliced open by prying apart the shells at the hinge and then severing the abductor muscle to reveal the internal organs. Two tissue samples from the mantle of each oyster were taken and placed into separate snap cap tubes with a 70% ethanol solution. Forceps and tweezers used to extract the tissue sample were sanitized after each oyster in a alconox, bleach, and water bath process to avoid DNA contamination. 64 tissue samples were collected.